Abstract
Imatinib mesylate (IM) has demonstrated to be highly effective in children with chronic myeloid leukemia (CML). The main issues remain the long-term side effects in pre-pubertal children and the poor compliance in adolescents. The aims of this study were: a) to evaluate the feasibility and efficacy of IM given intermittently to molecular responder (MR) CML children in chronic phase (CP), b) to reduce the long-term side effects of MR patients (pts) who started IM in pre-pubertal age, c) to improve compliance of poorly compliant adolescents in major MR (MMR).
Among CP-CML pts aged <18 years at diagnosis treated with IM at a dose of 340 mg/m2/day and with a follow-up ≥36 months, those with a persistent MR4.5-MR5 or in MR with long-term side effects, and those in MMR who were poorly compliant to continuous IM, were considered eligible for an intermittent IM (int-IM) administration. The intermittent schedule consisted of IM administered at the same dose for 3 weeks monthly (3 weeks on, 1 week off). Quantitative molecular analysis of the BCR-ABL1 transcript levels was carried out monthly on peripheral blood and every 3 months on bone marrow, along with cytogenetics. Long-term side effects (bone and mineral metabolism, growth rate and pubertal development) were regularly monitored. Re-treatment with IM given continuously or with other tyrosine kinase inhibitors (TKI) was planned if there was a loss of MMR in two consecutive samples or a cytogenetic relapse. IM discontinuation was considered for patients with a persistent MR4.5-MR5.
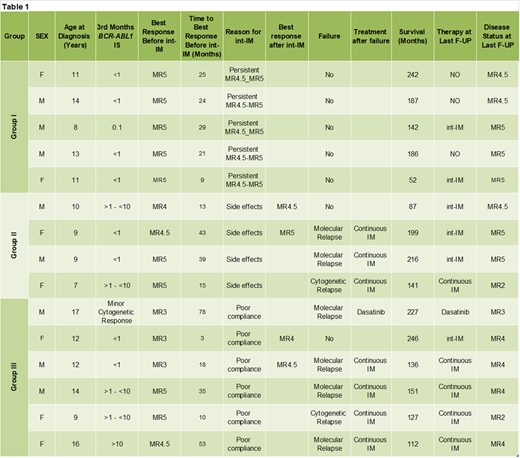
Fifteen of 58 CP-CML pts diagnosed between March 2001 and June 2015 received IM given intermittently for a median of 40 months(range: 14-86). Before starting int-IM, 5 pts had been in persistent MR4.5-MR5 for a median of 39 months (Group I), 4 pts had been in MR for a median of 28.5 months and had been suffering from long-term side effects (Group II), and 6 pts had been in MMR and were poorly compliant to treatment (Group III). Features and outcome of the 15 pts are shown in Table 1. Group I: 3/5 pts in MR4.5-MR5 discontinued int-IM after 16, 34 and 36 months and remain in continuous MR after 89, 90 and 107 months; 2 pts are still receiving int-IM for 14 and 36 months, respectively. Group II: 2/4 pts achieved a deeper MR (1 from MR4 to MR4.5 and 1 from MR4.5 to MR5) while on int-IM. However, 3 pts lost the response after 24 (cytogenetic relapse), 40 and 77 (molecular) months from the beginning of int-IM; all of them were successfully treated with IM given continuously. One pt is still receiving int-IM. Group III: 2/6 pts achieved a deeper MR (1 from MR3 to MR4.5 and 1 from MR3 to MR4) while on int-IM. However, 5/6 patients lost their response after a median time of 69 months (57-74). Four of them were treated with IM given continuously and 1 received dasatinib; all obtained a response. One, who achieved a deeper MR (MR3 to MR4), is still on int-IM after 86 months. Overall, 4/15 (26.7%) pts improved their molecular response while on int-IM and 3/15 pts (20%) successfully discontinued treatment. On the other hand, 8/15 pts (53.3%) failed int-IM after a median of 63 months (range 24-77) from the beginning (6 pts lost MMR and 2 pts had a cytogenetic relapse). No pt underwent a stem cell transplantation. Long-term side effects (bone metabolism, growth rate and pubertal development) progressively improved during IM given intermittently. At the last follow-up, 6 pts (4 MR5, 1 MR4.5, 1 MR3) are still receiving int-IM, 5 (3 MR4, 2 MR2) are receiving continuous IM, 3 (MR4.5-MR5) are treatment-free and 1 (MR3) is being treated with dasatinib. All patients are alive at a median time from diagnosis of 151 months (range 52-266).
Our experience suggests that an intermittent schedule of IM given 3 weeks a month could be an effective strategy before stopping IM in CP-CML children in persistent deep MR. Moreover, this approach is capable of improving the molecular response in poor compliant pts and is useful to reduce IM-related long-term side effects. The relatively high number of relapses in group III (5/6, 83%) is indicative of its poor efficacy in pts not compliant to IM given continuously. Based on these data, a prospective cooperative study has been planned.
Malaspina:Sapienza University, Rome: Other: Resident in Hematology. Rizzo:Sapienza University, Rome: Other: Resident in Hematology. Locatelli:bluebird bio: Consultancy; Bellicum: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Foà:CELGENE: Other: ADVISORY BOARD, Speakers Bureau; AMGEN: Other: ADVISORY BOARD; CELTRION: Other: ADVISORY BOARD; GILEAD: Speakers Bureau; NOVARTIS: Speakers Bureau; JANSSEN: Other: ADVISORY BOARD, Speakers Bureau; INCYTE: Other: ADVISORY BOARD; ABBVIE: Other: ADVISORY BOARD, Speakers Bureau; ROCHE: Other: ADVISORY BOARD, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal